MarkVCID Consortium Overview
Biomarkers for Vascular Contributions to Cognitive Impairment and Dementia (MarkVCID) is an NIH funded multisite consortium dedicated to validating promising predictive, diagnostic, target engagement and progression biomarkers of the brain small-vessel diseases involved in the vascular contribution to cognitive impairment and dementia (VCID).
NINDS, an External Advisory Committee, and interested non-governmental organizations provide ongoing advice and oversight to MarkVCID research projects. Learn more about MarkVCID1 (the first phase of the study) and findings here.
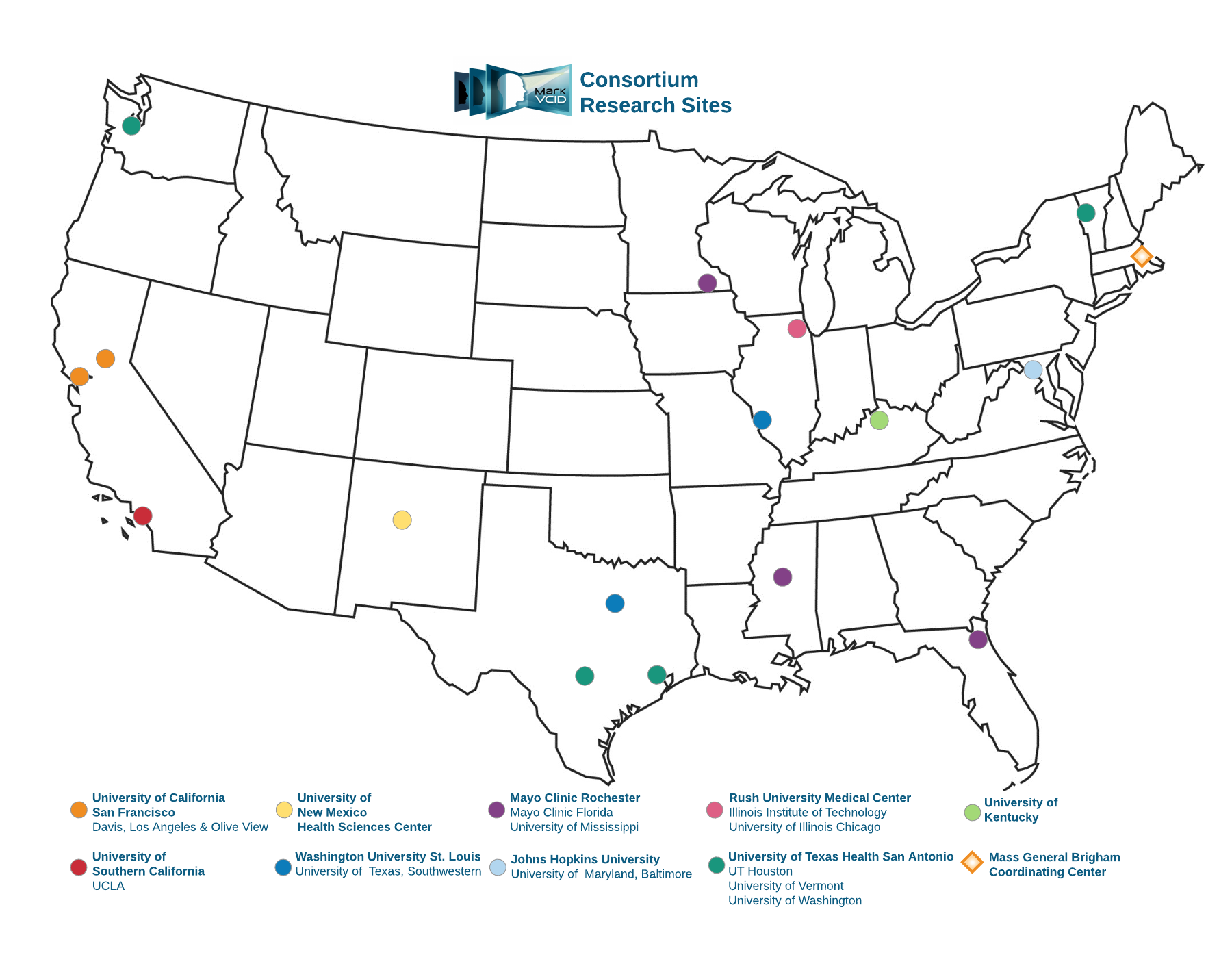
MarkVCID2 (the second phase of the study) consists of nine performance sites comprised of 15 US medical centers, a Coordinating Center, an External Advisory Committee, and NIH leadership. MGH serves as the Consortium’s Coordinating Center and is comprised of an Administrative Core, Data Core, and Biostatistical and Analytical Subcore providing participating research sites with a common support infrastructure that facilitates cross-site collaborations, oversees development of standardized common study procedures and data-collection methods, and manages consortium-wide data and analyses.
Research sites are charged with performing biological validation of candidate biomarkers that were selected in the first, instrumental validation phase, of MarkVCID. The Consortium’s five-year funding period is dedicated to each project site enrolling ≥200 diverse human subjects in the first 2 years with cognitive complaints and/or early symptomatic stages of cognitive impairment and dementia diagnoses potentially associated with cerebrovascular small vessel disease; then following enrolled subjects longitudinally through annual return visits. Throughout the duration of the study, sites will utilize harmonized data acquisition procedures to support biomarker validation.
Meet the Team
Age-related cognitive impairment and dementia represent one of the greatest risks to public health both in the US and globally. Alzheimer’s disease is the commonest cause of cognitive impairment, but diseases of the brain’s blood vessels—particularly the network of small blood vessels that supply all parts of the brain—have also been shown to be major contributors. Many medical trials have been conducted to find ways to prevent cognitive impairment due to small vessel diseases but have been hampered by the limited availability of “biomarkers” that identify which people should be treated, detect which disease pathways should be targeted, or indicate that a particular treatment is working.
NINDS leadership selected 5 candidate imaging and fluid-based biomarkers to undergo clinical validation in MarkVCID2 to the point of readiness for incorporation into future VCID interventional trials:
- MRI Arteriosclerosis (ARTS); kit lead: Konstantinos Arfanakis, PhD
- MRI Cerebrovascular Reactivity (CVR); kit lead: Hanzhang Lu, PhD
- MRI Peak Skeletonized Mean Diffusivity (PSMD); kit lead: Claudia Satizabal, PhD
- MRI Free Water (FW); kit leads: Pauline Maillard, PhD & Arvind Caprihan, MD
- Plasma Neurofilament Light (NfL); kit leads: Sudha Seshadri, MD & Claudia Satizabal, PhD
MarkVCID welcomes collaboration with investigators outside the Consortium. Publicly available resources include:
- Clinical/cognitive measure collection manuals
- Biospecimen collection Best Practices
- Imaging standard operating procedures
- Patient MRI protocols
- Imaging- and fluid-based biomarker kit protocols
Click here to navigate to the Consortium's Phase 2 Protocols & Resources.
Study Committees
To help accomplish its mission, Steering oversees six subcommittees compromised of experts from research sites and the coordinating center who guide and recommend consortium procedures:



